
The cohort is “followed up” retrospectively. The methodology is the same but the study is performed posthoc. These use data already collected for other purposes.

Where two cohorts are used, one group has been exposed to or treated with the agent of interest and the other has not, thereby acting as an external control. In single cohort studies those people who do not develop the outcome of interest are used as internal controls. Over a period of time the people in the sample are observed to see whether they develop the outcome of interest (that is, myocardial infarction).

The investigator then measures a variety of variables that might be relevant to the development of the condition. Prospective cohort studiesĪ group of people is chosen who do not have the outcome of interest (for example, myocardial infarction). The studies may be prospective or retrospective and sometimes two cohorts are compared. These are the best method for determining the incidence and natural history of a condition. In so doing it should become apparent why a given study used a particular research method and which method might best answer a particular clinical problem. In this article each of the three important observational research methods will be discussed with emphasis on their strengths and weaknesses. For example, a study showing that 80% of the Swedish population has blond hair, might be used to make a sensible prediction of the incidence of blond hair in other Scandinavian countries, but would be invalid if applied to most other populations.Įvery published study should contain sufficient information to allow the reader to analyse the data with reference to these key points. The question of external validity relates to the value of the results of the study to other populations-that is, the generalisability of the results. A study that is not internally valid should not be published because the findings cannot be accepted. For a study to be regarded as valid it must be shown that it has indeed demonstrated what it says it has. That is, the conclusions can be logically drawn from the results produced by an appropriate methodology. Missing data-how much are unavailable and why?Įxtraneous treatments-other interventions that may have affected some but not all of the subjects.Ĭonfounding factors-Are there other variables that might influence the results?Īppropriate analysis-Have appropriate statistical tests been used?Īll studies should be internally valid. Quality control-has the methodology been rigorously adhered to?Ĭompliance-did all patients comply with the study?ĭrop outs-how many failed to complete the study? Reproducibility-can the results be repeated or is there a reason to suspect they may be a “one off”?īlinded-were the investigators or subjects aware of their subject/control allocation? Validity-are the measurements used regarded as valid by other investigators? The source of the controls should be explained-are they from the same population as the sample?Īre the controls matched or randomised-to minimise bias and confounding. The control group should be easily identifiable. The number of patients lost to follow up should be stated and explanations given. The sampling method should be described and the sample size should be justified.Įntry criteria and exclusions should be stated and justified. The source of the sample should be stated. The sample should accurately reflect the population from which it is drawn. The aim of the study should be clearly stated. They are often used to generate hypotheses that can then be studied via prospective cohort or other studies.

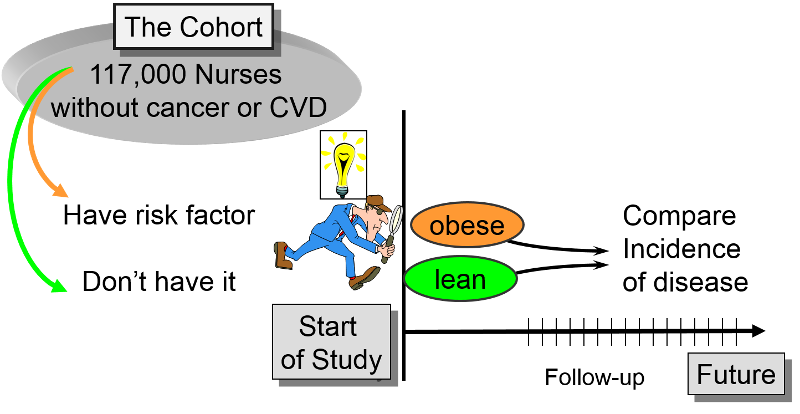
They seek to identify possible predictors of outcome and are useful for studying rare diseases or outcomes. Case controlled studies compare groups retrospectively. They are relatively quick and easy but do not permit distinction between cause and effect. Cross sectional studies are used to determine prevalence. Because they measure events in chronological order they can be used to distinguish between cause and effect. Cohort studies are used to study incidence, causes, and prognosis.
RETROSPECTIVE COHORT STUDY DEFINITION TRIAL
Often these studies are the only practicable method of studying various problems, for example, studies of aetiology, instances where a randomised controlled trial might be unethical, or if the condition to be studied is rare. Cohort, cross sectional, and case-control studies are collectively referred to as observational studies.


 0 kommentar(er)
0 kommentar(er)
